A Multidisciplinary Approach to Antibiotic Resistance

Background
Antibiotic resistance has become a global threat to public health due to the emergence of extensively antibiotic resistant bacteria and the sharp decline in antibiotic discovery. In the most extreme cases, antibiotic resistance can lead to untreatable infections that result in persistent infections and high mortality rates.
However, persistent infections are often caused by antibiotic susceptible bacteria, including multidrug-resistant bacteria that in most cases remain susceptible to antibiotics of last resort, illustrating the limitations of available antibiotics in the complex environment of the host and the need to develop new approaches to treat bacterial infections more effectively.
Klebsiella pneumoniae
Most projects in the lab employ Klebsiella pneumoniae (Kp) to study various aspects of persistent infections. Kp is one of the most concerning antibiotic resistance threats and a common cause of hospital-acquired infections. Particularly concerning is the rise of extremely antibiotic resistant strains that have become resistant to carbapenem antibiotics and colistin, antibiotics of last resort to treat multidrug resistant infections.
In spite of globally occurring morbidity and mortality in the clinic, the pathogenesis of the most common strains remain enigmatic. Basic virulence factors point to a defensive strategy that appears to promote persistent infection and virulence in patients with weakened immune systems and co-morbidities. How are classical Kp strains able to cause disease? What is the basis for the epidemic success of clonal groups that are associated with multidrug-resistance?
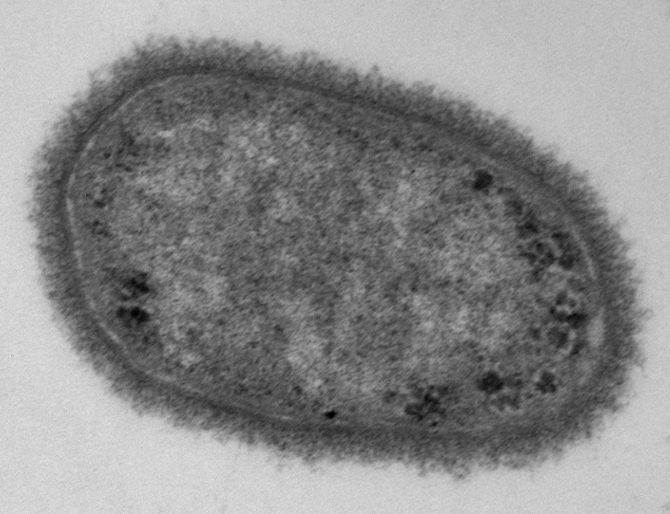
Electron microscopy image of a classical Klebsiella pneumoniae strain
Adaptive evolution
Mutations that are positively selected in patients have the potential to reveal unrecognized pathogenesis pathways in hospital-associated pathogens, including their ability to cause persistent infections. Adaptive mutations can result in purifying selection or lead to diversification of the microbial population, in which a clade emerges and continues to evolve.
We have found that diversification occurs frequently in microbial populations from multidrug resistant infections and that the mutations can reveal selective pressures on infection pathways that may have been overlooked, such as the ability of Kp to persist for days in LAMP1 vacuoles of bladder epithelial cells and tolerate lethal concentrations of antibiotics. How is Kp evolving in patients? Can adaptive evolution reveal more insights into the pathogenesis and antibiotic treatment failure of Kp ?
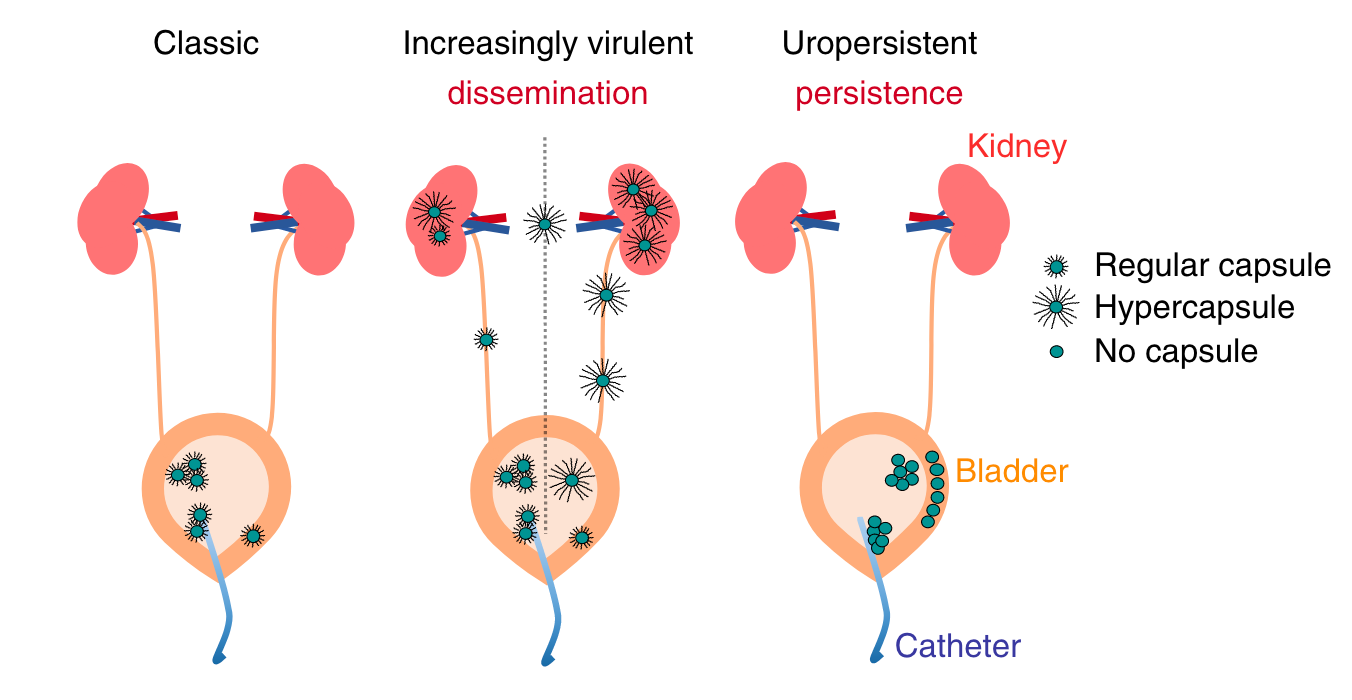
Kp repeatedly evolves virulence and persistence in antibiotic-resistant infections (Ernst et al. 2020)
Persistence
Persistent infections, including latent infections, provide a basis for within-patient evolution and recurrent infections. The lab is thus interested in identifying host-pathogen interactions that promote persistent infections to gain a better understanding of the basis of disease progression that may not only include disease exacerbation and resolution. We are particularly interested in the ability of common hospital-associated pathogens to survive intracellularly, which is relevant in the context of urinary tract infections and involves general metabolic and pathological features that promote antibiotic tolerance.
Virulence factors that are usually associated with intracellular survival have not been identified in Kp or UPEC. Previous studies with other pathogens and our preliminary studies with Kp suggest that host pathogen interactions that promote intracellular survival also lead to antibiotic tolerance. How are Kp and UPEC able to survive in intracellular compartments? What allows them to overcome cell autonomous immunity and antibiotics?
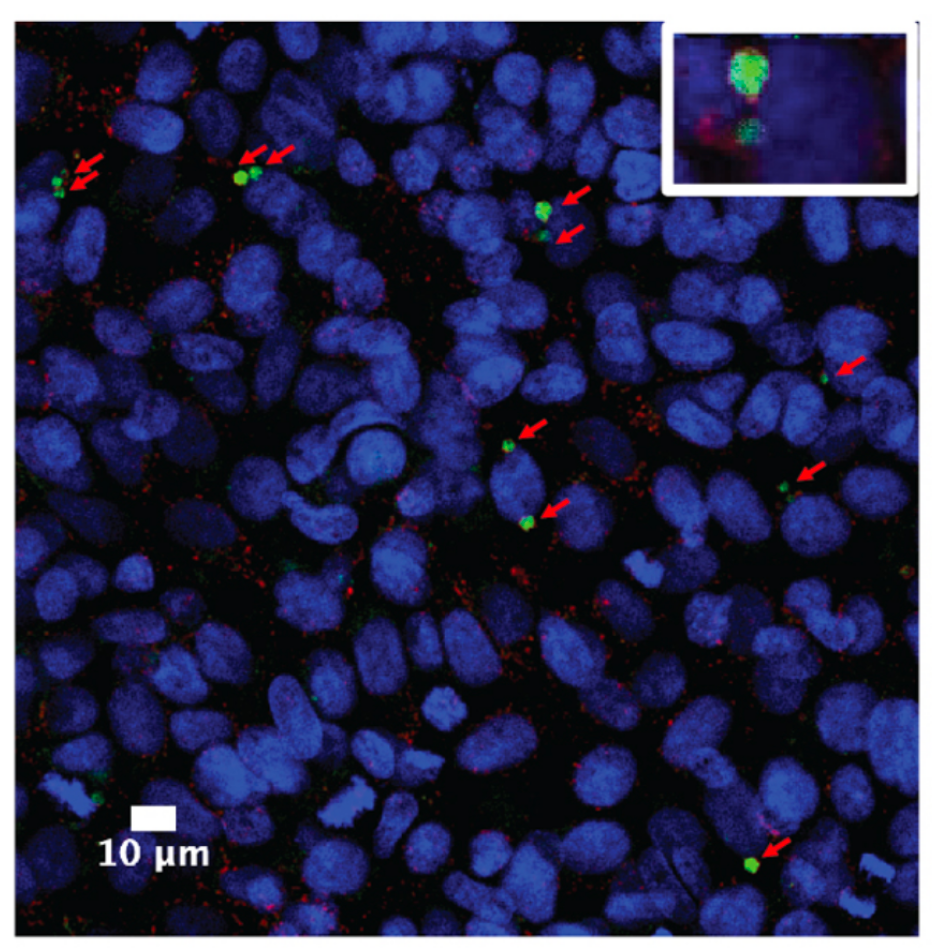
Confocal microscopy image of intracellular Kp (green)persisting for several days in LAMP1 positive vacuoles (red) of bladder epithelial cells
Towards new anti-infective strategies
The emergence of extensively drug resistant bacteria and the difficulty of treating persistent bacterial infections requires the development of new antibiotics and antimicrobial strategies.
After identifying several host targeting compounds that clear intracellular infections, we have become particularly interested in the intersection of host metabolism and cell autonomous immunity. Can insights into host pathogen interactions lead to new antimicrobial strategies? Can novel antiinfectives be identified in high-throughput infection screens?
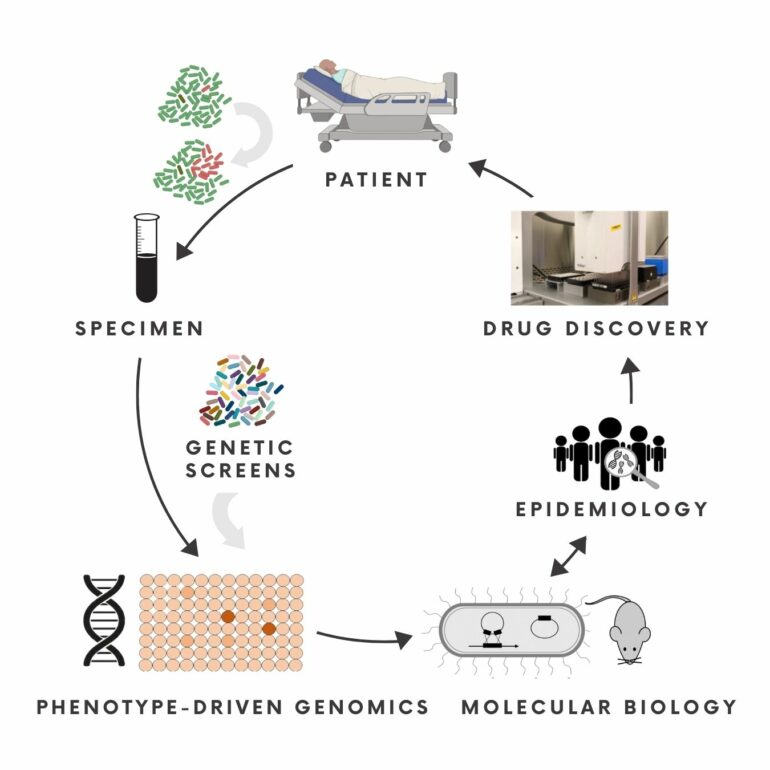
A Multidisciplinary Approach to Antibiotic Resistance
The Ernst Lab works at the interface of molecular microbiology, genomics, epidemiology and chemical biology to identify, investigate and target unrecognised pathways in persistent and multidrug-resistant infections.
To this end, the lab conducts mechanistic investigations, develops genetic screens in infection related settings, and studies the adaptive evolution of pathogenicity and antibiotic tolerance to identify in vivo essential pathways in the context of persistent and multidrug-resistant infections. Insights from these studies provide a basis to explore unconventional antimicrobial strategies.
Background
Antibiotic resistance has become a global threat to public health due to the emergence of extensively antibiotic resistant bacteria and the sharp decline in antibiotic discovery. In the most extreme cases, antibiotic resistance can lead to untreatable infections that result in persistent infections and high mortality rates.
However, persistent infections are often caused by antibiotic susceptible bacteria, including multidrug-resistant bacteria that in most cases remain susceptible to antibiotics of last resort, illustrating the limitations of available antibiotics in the complex environment of the host and the need to develop new approaches to treat bacterial infections more effectively.
Klebsiella pneumoniae
Most projects in the lab employ Klebsiella pneumoniae (Kp) to study various aspects of persistent infections. Kp is one of the most concerning antibiotic resistance threats and a common cause of hospital-acquired infections. Particularly concerning is the rise of extremely antibiotic resistant strains that have become resistant to carbapenem antibiotics and colistin, antibiotics of last resort to treat multidrug resistant infections.
In spite of globally occurring morbidity and mortality in the clinic, the pathogenesis of the most common strains remain enigmatic. Basic virulence factors point to a defensive strategy that appears to promote persistent infection and virulence in patients with weakened immune systems and co-morbidities. How are classical Kp strains able to cause disease? What is the basis for the epidemic success of clonal groups that are associated with multidrug-resistance?

Electron microscopy image of a classical Klebsiella pneumoniae strain
Adaptive evolution
Mutations that are positively selected in patients have the potential to reveal unrecognized pathogenesis pathways in hospital-associated pathogens, including their ability to cause persistent infections. Adaptive mutations can result in purifying selection or lead to diversification of the microbial population, in which a clade emerges and continues to evolve.
We have found that diversification occurs frequently in microbial populations from multidrug resistant infections and that the mutations can reveal selective pressures on infection pathways that may have been overlooked, such as the ability of Kp to persist for days in LAMP1 vacuoles of bladder epithelial cells and tolerate lethal concentrations of antibiotics. How is Kp evolving in patients? Can adaptive evolution reveal more insights into the pathogenesis and antibiotic treatment failure of Kp ?

Kp repeatedly evolves virulence and persistence in antibiotic-resistant infections (Ernst et al. 2020)
Persistence
Persistent infections, including latent infections, provide a basis for within-patient evolution and recurrent infections. The lab is thus interested in identifying host-pathogen interactions that promote persistent infections to gain a better understanding of the basis of disease progression that may not only include disease exacerbation and resolution. We are particularly interested in the ability of common hospital-associated pathogens to survive intracellularly, which is relevant in the context of urinary tract infections and involves general metabolic and pathological features that promote antibiotic tolerance.
Virulence factors that are usually associated with intracellular survival have not been identified in Kp or UPEC. Previous studies with other pathogens and our preliminary studies with Kp suggest that host pathogen interactions that promote intracellular survival also lead to antibiotic tolerance. How are Kp and UPEC able to survive in intracellular compartements? What allows them to overcome cell autonomous immunity and antibiotics?

Confocal microscopy image of intracellular Kp (green)persisting for several days in LAMP1 positive vacuoles (red) of bladder epithelial cells
Towards new anti-infective strategies
The emergence of extensively drug resistant bacteria and the difficulty of treating persistent bacterial infections requires the development of new antibiotics and antimicrobial strategies.
After identifying several host targeting compounds that clear intracellular infections, we have become particularly interested in the intersection of host metabolism and cell autonomous immunity. Can insights into host pathogen interactions lead to new antimicrobial strategies? Can novel antiinfectives be identified in high-throughput infection screens?